Advanced Search
Search Results
The CSIR assumed its role as host of South Africa’s Centre for the Fourth Industrial Revolution (C4IR South Africa) in 2021.
Solar Photovoltaic (PV) Quality and Reliability Testing Facility
The CSIR photovoltaic module quality and reliability lab
The CSIR’s photovoltaic (PV) module quality and reliability testing laboratory – a first of its kind for Africa – includes world-class equipment for localising accelerated reliability stress testing on PV modules. This ensures that only high-quality modules that are suitable to the unique South African climate are developed and installed.
The CSIR’s accelerated stress tests provide quantitative metrics for evaluating the performance and reliability of solar PV modules over time. “The tests accelerate the failures in poorly constructed modules that can otherwise take years to occur naturally in the field, thereby helping to ensure reliable solar PV plant performance. Like a referee on the field, the lab tests ensure that the suppliers play fair and provide only high-quality and reliable modules”, says CSIR solar PV expert, Lawrence Pratt.
The environmental and mechanical stress testing forms the foundation for the pre-qualification of new concepts, certification of new PV modules and reliability testing of existing PV technology. “We have the equipment, experience and expertise to conduct testing on most of the common PV technologies, including crystalline, thin film, high capacitance and bifacial modules,” explains Pratt.
The PV module quality and reliability testing services are designed around specialised equipment for PV module extended reliability. The specialised equipment includes environmental chambers to simulate the real-world environmental conditions listed below:
- Thermal cycling of modules from -40 C to 85 C for 200, 400, and 600 cycles to simulate stress from thermal expansion and contraction.
- Humidity freeze testing: 85 C / 85% RH for 20 hours and a -40 C freeze for 10, 20, and 30 cycles to stress the lamination and adhesion strength of the PV module.
- Damp heat testing: 85 C / 85% RH for 1 000 hours, 2 000 hours, with and without electrical bias to stress the adhesion and insulation of the PV module.
- Mechanical load testing: static and dynamic load for simulating transportation, installation, and wind loads that may lead to broken cells and weakened interconnections.
The lab also operates specialised characterisation equipment to measure the electrical performance and safety aspects of the PV module. Pratt points out that the sun simulator outputs an equivalent dose of energy nearly identical to the sun’s energy and spectrum, but only for a fraction of a second. “This is just enough time to accurately and precisely record the current, voltage and power output of the PV module across various temperatures (15 °C and 75 °C) and light intensities (200 W/m2 to 1100 W/m2)”, he says.
The sun simulator is used to compare the output of the PV module as measured at the factory against an independent standard. The sun simulator is also critical to quantify the impact of the accelerated stress tests described above. Finally, the high potential electrical tester helps to identify failures in the insulation resulting from accelerated stresses. Failures in electrical insulation indicate a potential risk to the safe and reliable performance of PV modules over time.
The PV team at the CSIR also offers outdoor test services for module-level performance testing, soiling studies, degradation studies and small-scale string inverter system testing in a real-world environment.
Ventilators
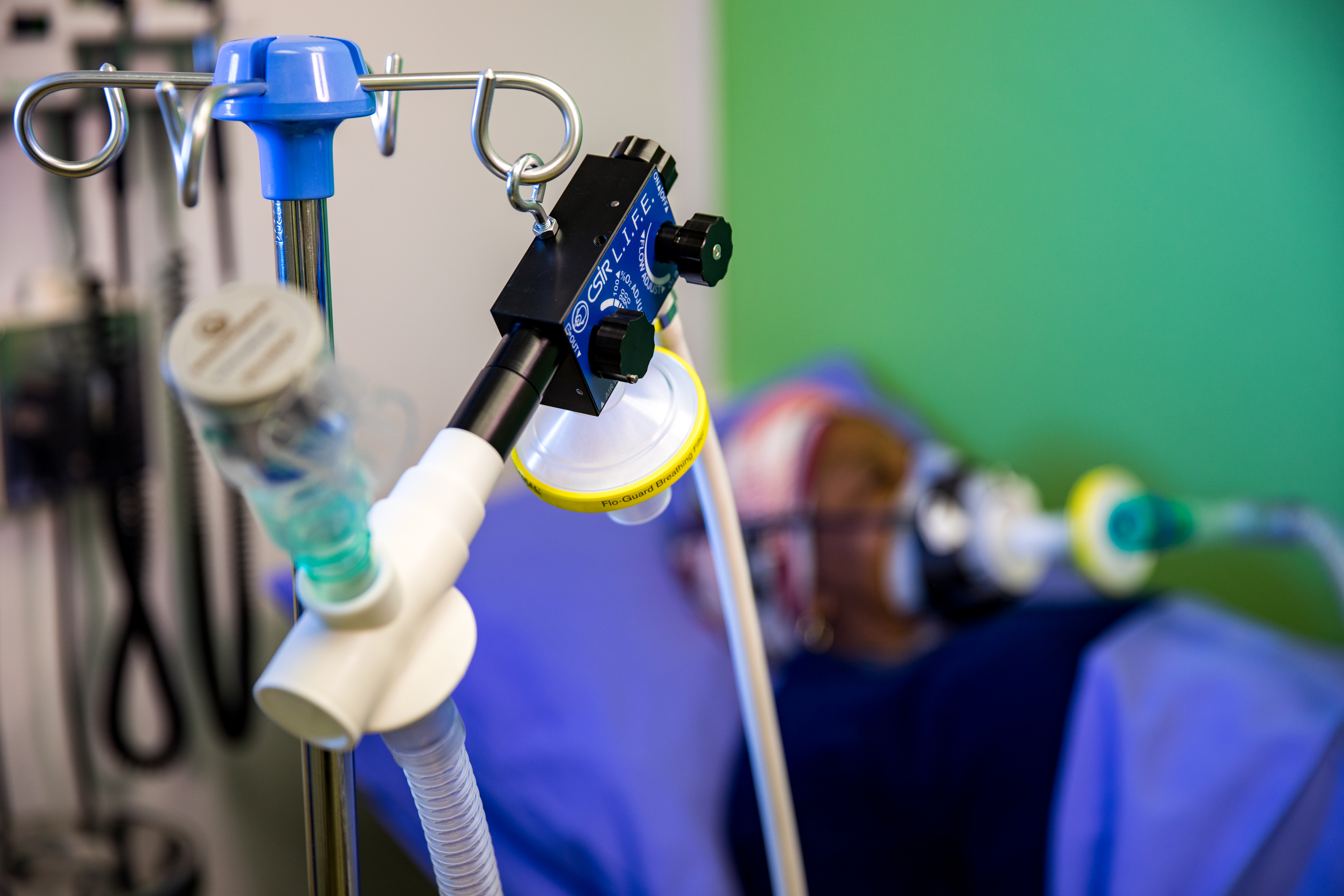
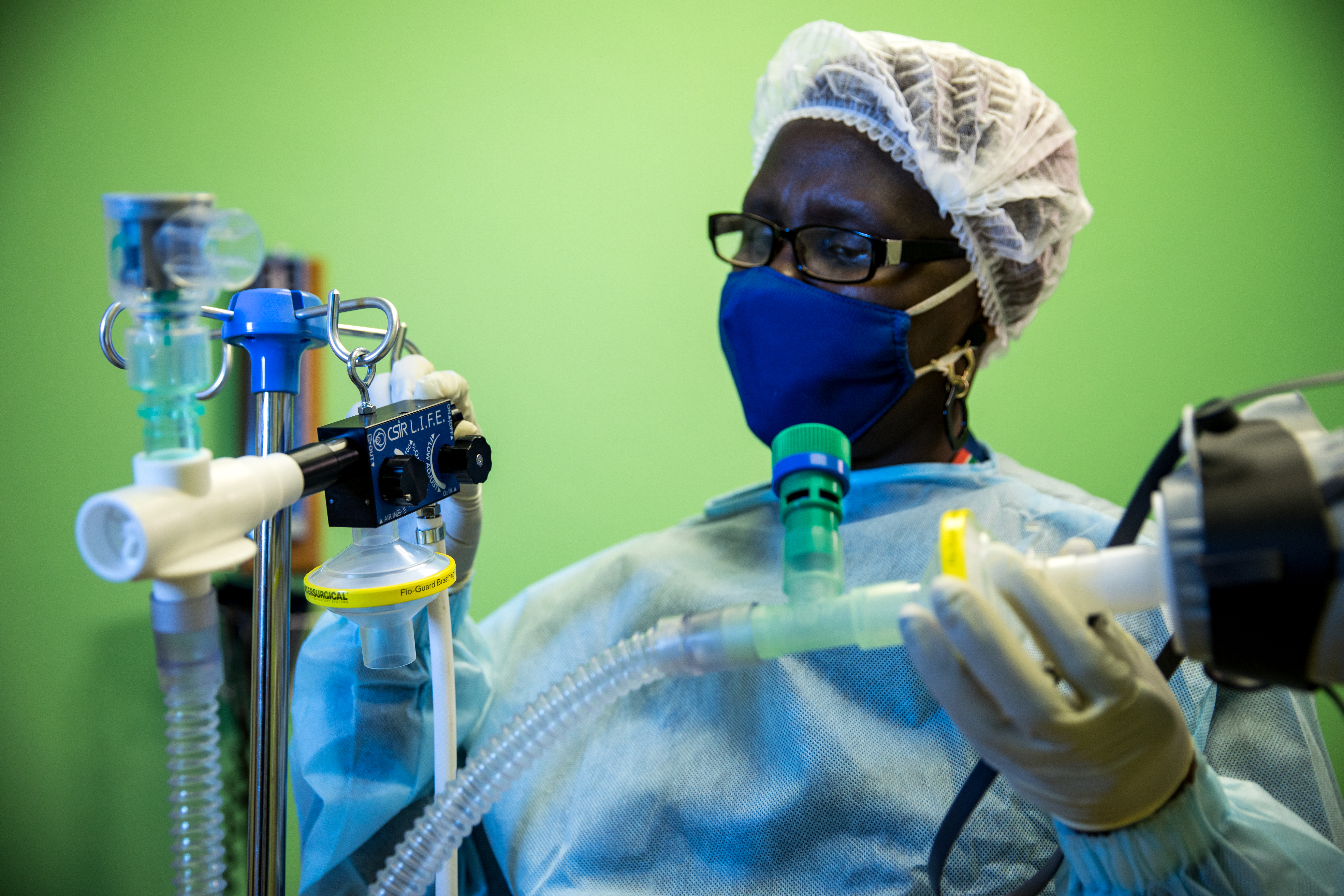
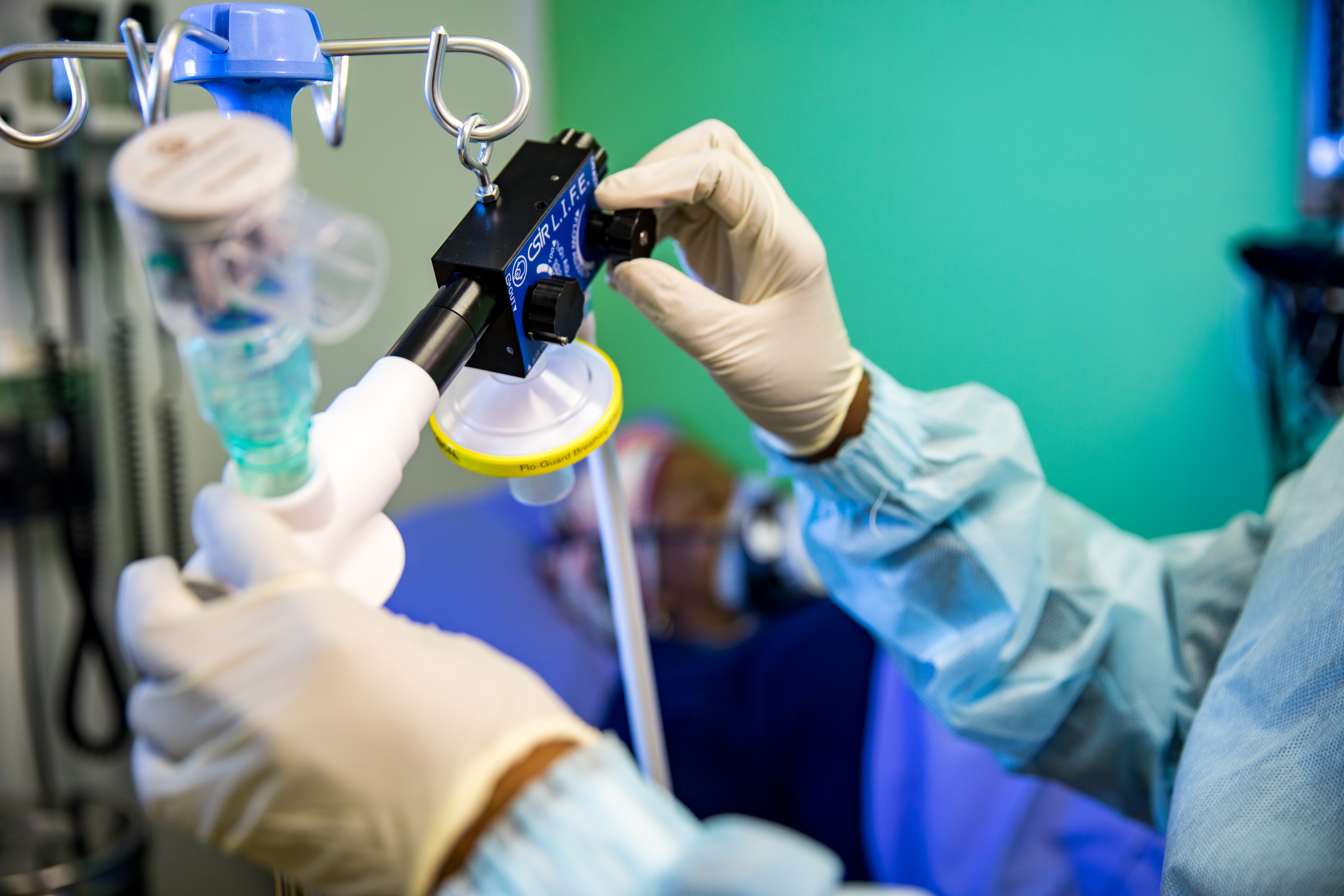
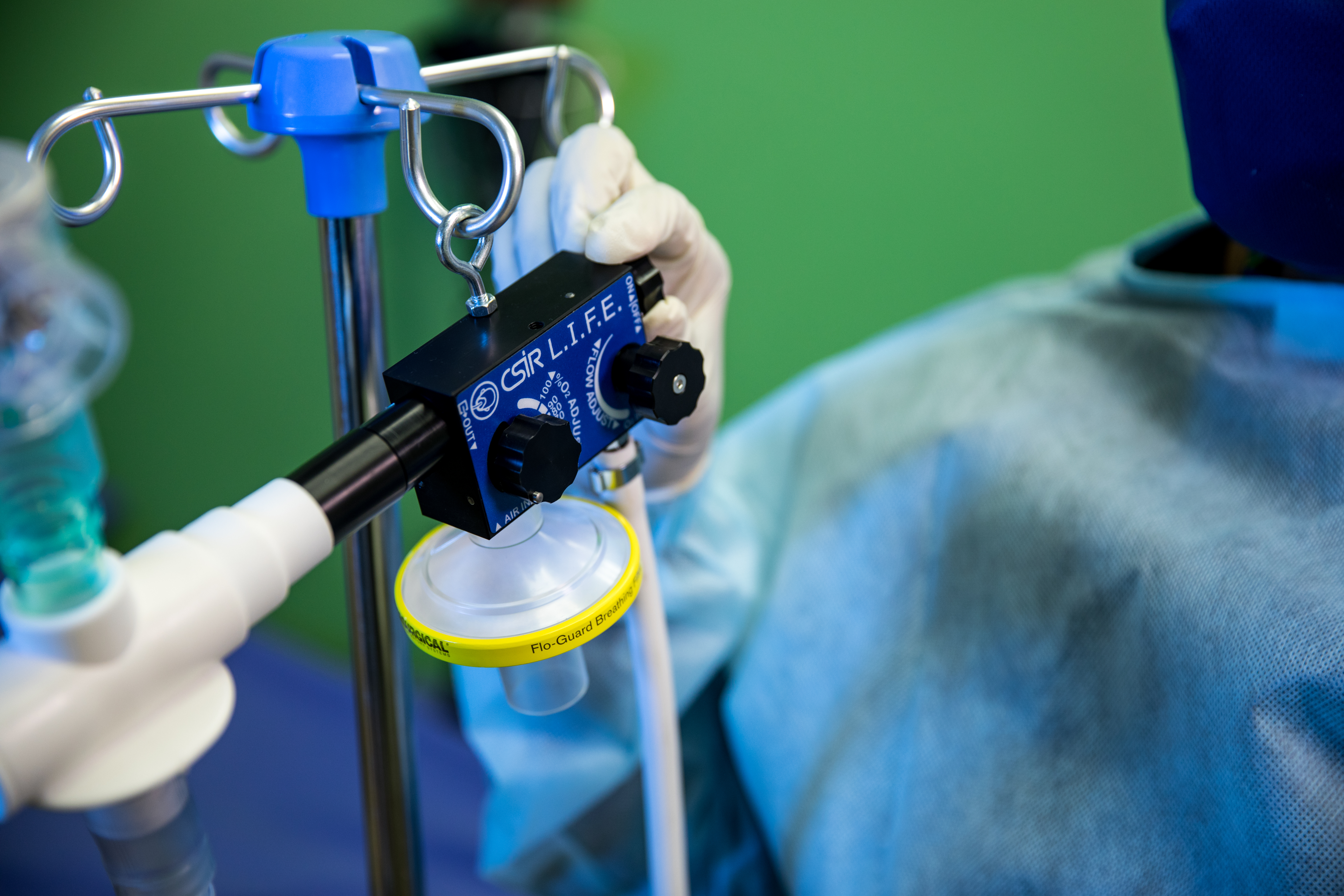
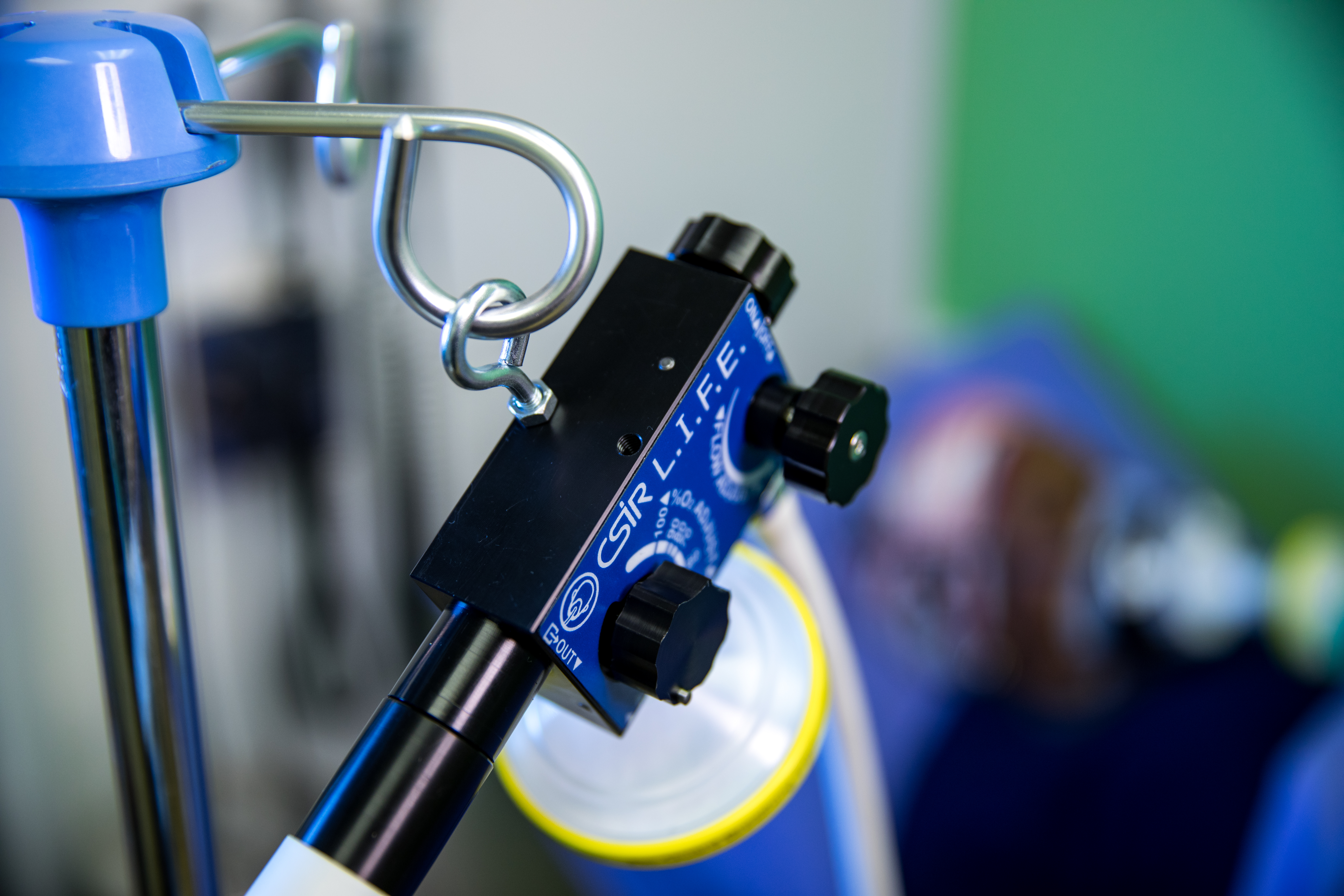
CSIR L.I.F.E. - Acting swiftly to touch lives through innovation
The digital design and production of a local ventilator to support the national response to the Covid-19 pandemic took place in under 3 months, and saw the CSIR take the lead in this collaborative effort to swiftly touch lives through innovation.
Under the auspices of the Department of Trade, Industry and Competition (the dtic), the CSIR worked closely with a number of local partners to develop the Continuous Positive Airway Pressure (CPAP) device that uses an innovative design to provide a mild level of oxygenated air pressure to keep the airways open and, thus, assist with breathing. Through this project, a total of 18 000 ventilators were produced supplying oxygen to Covid-19 patients showing respiratory distress.
Under the project name, ‘CSIR L.I.F.E.’ (Lung Inspiratory Flow Enabler), the innovative system uses standard, hospital-grade oxygen supply, and features easy-to-use, on-device flow gages to adjust Fraction of Inspired Oxygen in steps of 10% oxygenation.
The development forms part of government’s National Ventilator Project (NVP), and is supported by the Solidarity Fund. “While ensuring that we achieve this in a short period of time, we had to ensure that we follow a rigorous, documented product lifecycle methodology that would ensure scalable manufacturing, as well as compliance and licensing under the South African Health Products Regulatory Authority (SAHPRA) and guidelines of the World Health Organization,” says Ajith Gopal, Executive Manager of CSIR Future Production: Manufacturing.
The CSIR thermal laboratory
The water and health microbiology research facility is key for research on the presence of microorganisms in water and how they impact human health.
The CSIR provides capability development and management decision support for clients in the safety and security cluster.
Secure Identity Framework
CSIR engineers a secure identity framework towards the development of the SMART ID card in South Africa
Responding to its objective to conduct research, development and innovation that supports the development of a capable state, the CSIR collaborated with the Department of Home Affairs to design and develop a secure identity framework that enabled the department to issue South Africans with a new Smart ID card embedded with security features aimed at significantly reducing potential forgery.
“This innovation is characterised by a multiapplication contactless card that securely stores the biographic and biometric data of the applicant during the personalisation and printing of the card,” explains CSIR information security expert, Samuel Lefophane. The secure identity framework enables identification, verification and authentication for e-government and e-commerce services, thus allowing the transition of government services from manual to digital.
The Smart ID card was adopted to reduce fraudulent activities that were prevalent in the era of the green ID book. Its development also presented benefits for both the public and private sectors, as they are now able to conduct identity verification for their customers employing improved and more reliable processes. The functionalities that are embedded in the Smart ID card are aligned with international standards, taking into consideration the unique needs of South Africa.
Some of the institutions that are likely to benefit from this CSIR technology within the private and public sectors are the Department of Health, Department of Community Safety, the South African Social Security Agency, the Traffic Department, Statistics South Africa, the Independent Electoral Commission, banks, insurance companies and many more.
SARS-COV-2 diagnostic PCR test kit
CSIR and CapeBio lead development of local SARS-COV-2 diagnostic PCR test kit
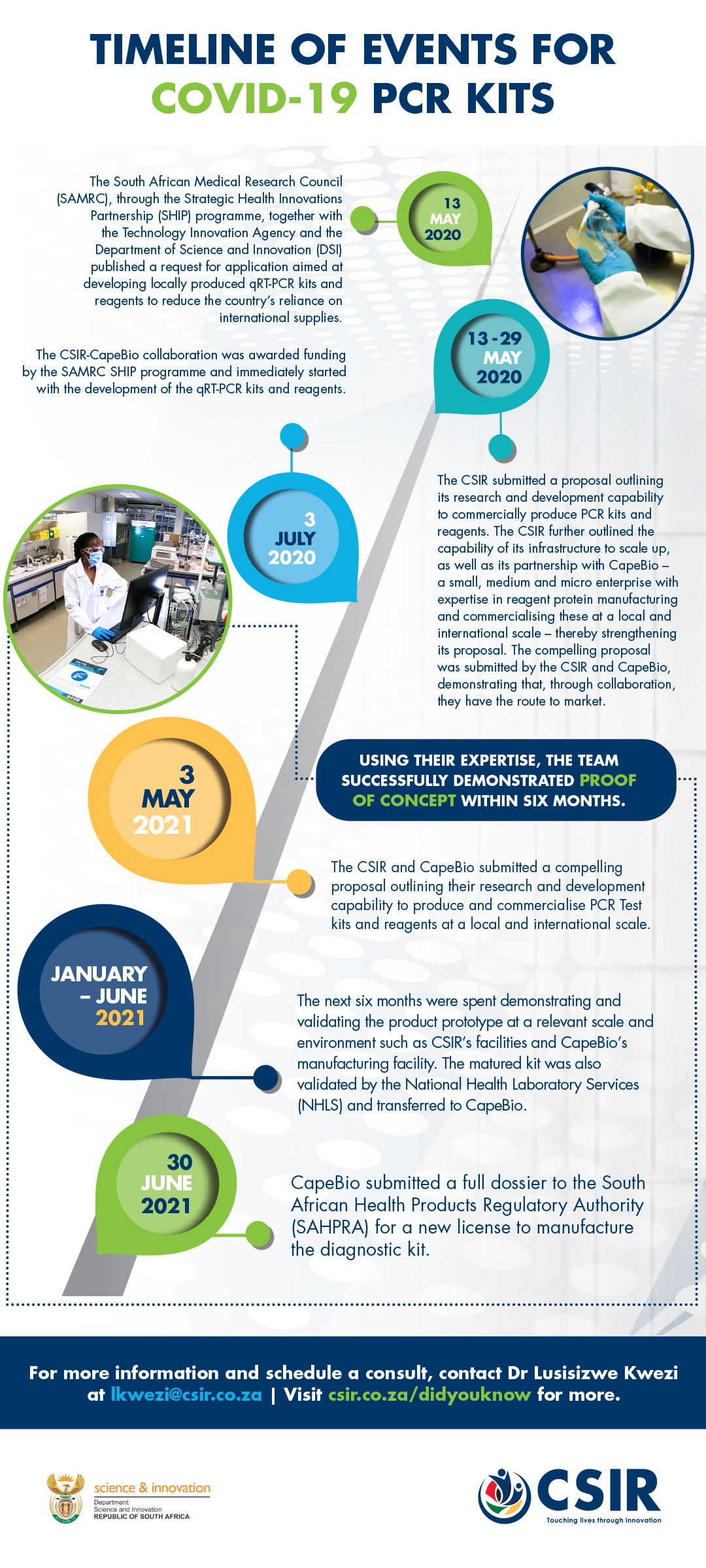
An overburdened demand, coupled with a worldwide shortage of rapid Covid-19 test kits, prompted the CSIR and CapeBio to demonstrate their expertise in biomanufacturing as a solution to provide the country, and the region, with a locally developed PCR test kit for Covid-19.
In early 2020, the CSIR, in collaboration with CapeBio, demonstrated the efficiency of the biomanufacturing process for two enzymes, which were combined to formulate a locally produced one-step Covid-19 diagnostic assay.
The diagnostic assay has been validated in a clinical setting and proven to have the capability to detect coronavirus 2 (SARS CoV-2)-specific genetic biomarkers. Furthermore, the single-step format of the diagnostic assay reduces the turnaround time of tests and assists in managing and monitoring the spread of SARS CoV-2.
Additionally, external evaluation by the National Health Laboratory Service was passed, and the South African Health Products Regulatory Authority subsequently licensed Cape Bio to manufacture the diagnostic kit at their facility in Centurion.
This significant milestone, which took place in under a year, resulted in the kit hitting the local market in August 2021. According to CapeBio CEO, Daniel Ndima, at full operational capacity, the company is able to produce up to 5 000 kits a day, with each kit providing 1 000 tests.
This is a clear demonstration that the CSIR, through its industrialisation strategy and commitment to assisting new and emerging small, medium and micro enterprises, is touching lives through innovative biomanufacturing processes.
The research and development funded by the South African Medical Research Council and the Technology Innovation Agency enables a faster response in terms of active case identification, quarantine and contact tracing. Additionally, the localisation of the production of these reagents continues to increase accessibility to locally produced diagnostic kits – a very significant milestone that will reduce the country’s reliance on international suppliers.
Related Information
The CSIR’s cybersecurity systems research group provides operational effectiveness on a national and organisational level through the operalisation of cybersecurity centric solutions and capabiliti