Advanced Search
Search Results
The Council for Scientific and Industrial Research (CSIR) congratulates Dr Patience Mthunzi-Kufa, Research Group Leader for Biophotonics at the CSIR, for being appointed by President Cyril Ramaphos
The CSIR provides capability development and management decision support for clients in the safety and security cluster.
Secure Identity Framework
CSIR engineers a secure identity framework towards the development of the SMART ID card in South Africa
Responding to its objective to conduct research, development and innovation that supports the development of a capable state, the CSIR collaborated with the Department of Home Affairs to design and develop a secure identity framework that enabled the department to issue South Africans with a new Smart ID card embedded with security features aimed at significantly reducing potential forgery.
“This innovation is characterised by a multiapplication contactless card that securely stores the biographic and biometric data of the applicant during the personalisation and printing of the card,” explains CSIR information security expert, Samuel Lefophane. The secure identity framework enables identification, verification and authentication for e-government and e-commerce services, thus allowing the transition of government services from manual to digital.
The Smart ID card was adopted to reduce fraudulent activities that were prevalent in the era of the green ID book. Its development also presented benefits for both the public and private sectors, as they are now able to conduct identity verification for their customers employing improved and more reliable processes. The functionalities that are embedded in the Smart ID card are aligned with international standards, taking into consideration the unique needs of South Africa.
Some of the institutions that are likely to benefit from this CSIR technology within the private and public sectors are the Department of Health, Department of Community Safety, the South African Social Security Agency, the Traffic Department, Statistics South Africa, the Independent Electoral Commission, banks, insurance companies and many more.
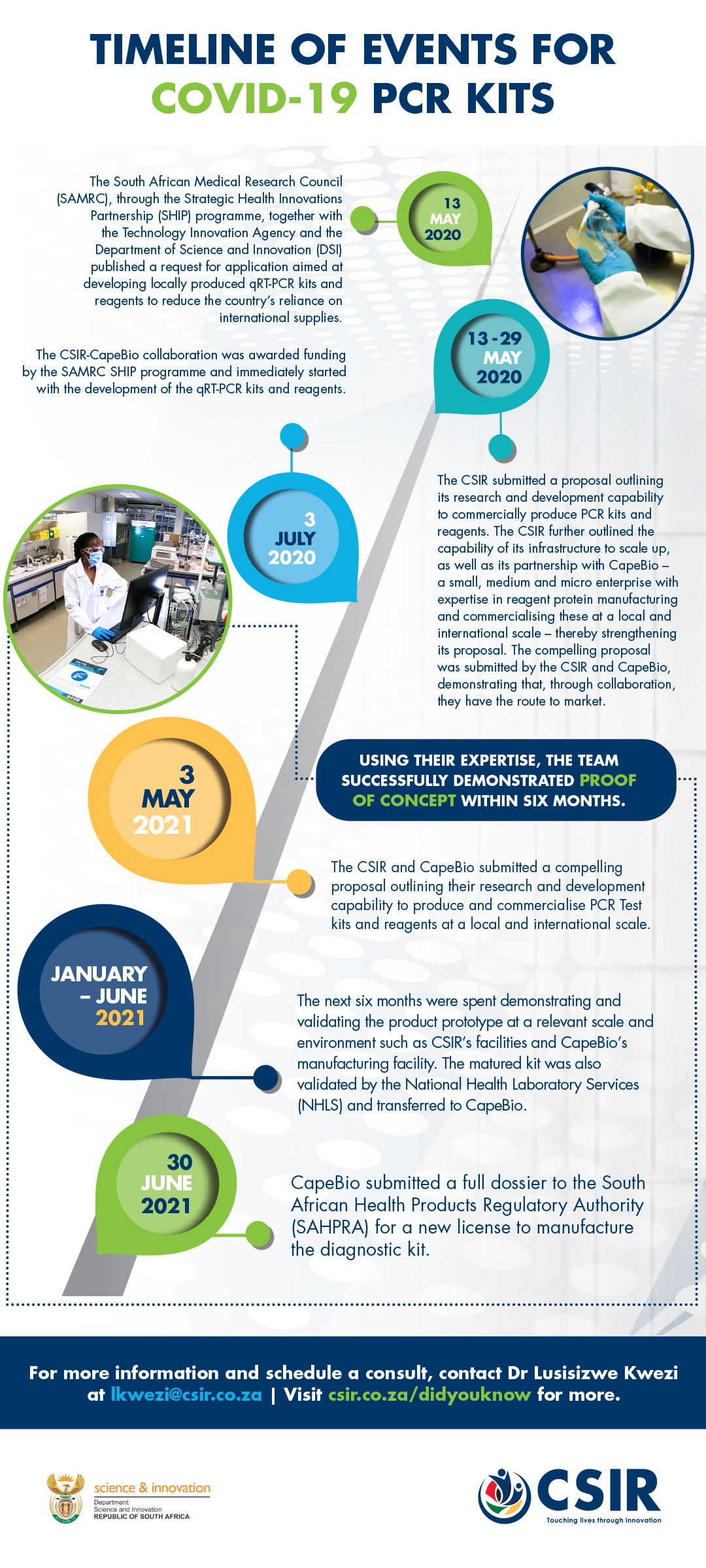
SARS-COV-2 diagnostic PCR test kit
CSIR and CapeBio lead development of local SARS-COV-2 diagnostic PCR test kit
An overburdened demand, coupled with a worldwide shortage of rapid Covid-19 test kits, prompted the CSIR and CapeBio to demonstrate their expertise in biomanufacturing as a solution to provide the country, and the region, with a locally developed PCR test kit for Covid-19.
In early 2020, the CSIR, in collaboration with CapeBio, demonstrated the efficiency of the biomanufacturing process for two enzymes, which were combined to formulate a locally produced one-step Covid-19 diagnostic assay.
The diagnostic assay has been validated in a clinical setting and proven to have the capability to detect coronavirus 2 (SARS CoV-2)-specific genetic biomarkers. Furthermore, the single-step format of the diagnostic assay reduces the turnaround time of tests and assists in managing and monitoring the spread of SARS CoV-2.
Additionally, external evaluation by the National Health Laboratory Service was passed, and the South African Health Products Regulatory Authority subsequently licensed Cape Bio to manufacture the diagnostic kit at their facility in Centurion.
This significant milestone, which took place in under a year, resulted in the kit hitting the local market in August 2021. According to CapeBio CEO, Daniel Ndima, at full operational capacity, the company is able to produce up to 5 000 kits a day, with each kit providing 1 000 tests.
This is a clear demonstration that the CSIR, through its industrialisation strategy and commitment to assisting new and emerging small, medium and micro enterprises, is touching lives through innovative biomanufacturing processes.
The research and development funded by the South African Medical Research Council and the Technology Innovation Agency enables a faster response in terms of active case identification, quarantine and contact tracing. Additionally, the localisation of the production of these reagents continues to increase accessibility to locally produced diagnostic kits – a very significant milestone that will reduce the country’s reliance on international suppliers.
Related Information
In 2012, the Department of Science and Technology (DST) and the CSIR embarked on a process to develop a Waste Research, De
The CSIR is home to leading aeronautical research and development.
The CSIR’s cybersecurity systems research group provides operational effectiveness on a national and organisational level through the operalisation of cybersecurity centric solutions and capabiliti
The CSIR and Much Asphalt Pty Ltd used locally available micro-fillers and recycled tyres to pave a 200-m long section of road as part of a controlled trial in Roodepoort, Gauteng.