Focus areas
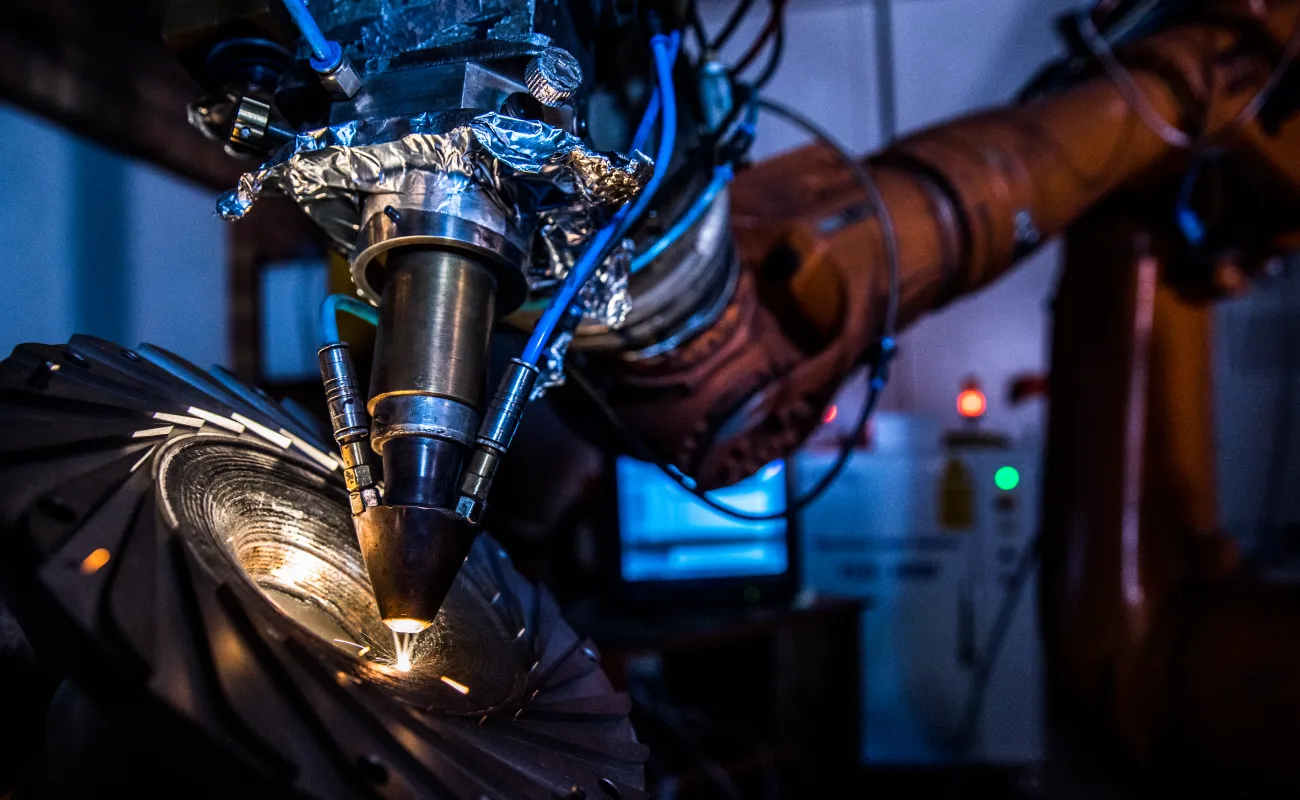
Laser technologies
The CSIR houses a critical core of laser expertise and scientific infrastructure for laser-related research and development, product development and industrial services across a wide range of sectors – from mining and health to defence, manufacturing, communication and power generation.We also support laser-related research and development at universities and other research institutions, locally and across the continent.
View more
Materials and manufacturing
We design manufacturing processes focused on speciality metals, alloys and next-generation composite materials. Our expertise includes structural design and analysis, as well as advanced casting and powder metallurgy technologies such as atomisation, hot isostatic pressing, metal injection moulding and press-and-sinter technologies.
View more
Robotics and smart manufacturing
Disruptive fourth industrial revolution (4IR) technologies hold significant potential for the local manufacturing industry to localise and streamline production, enhance skills and efficiency and boost global competitiveness. We have expertise in industrial robotics and sensors, industrial artificial intelligence, augmented reality and future smart production systems.
View more
Sensor systems
We develop customised sensor systems for inspection and monitoring purposes. These include sound and gas detection, underwater sensor systems for defence applications and ultrasonic technologies used in medical diagnostics.
View more

