What we do
Contact information:
Highlights

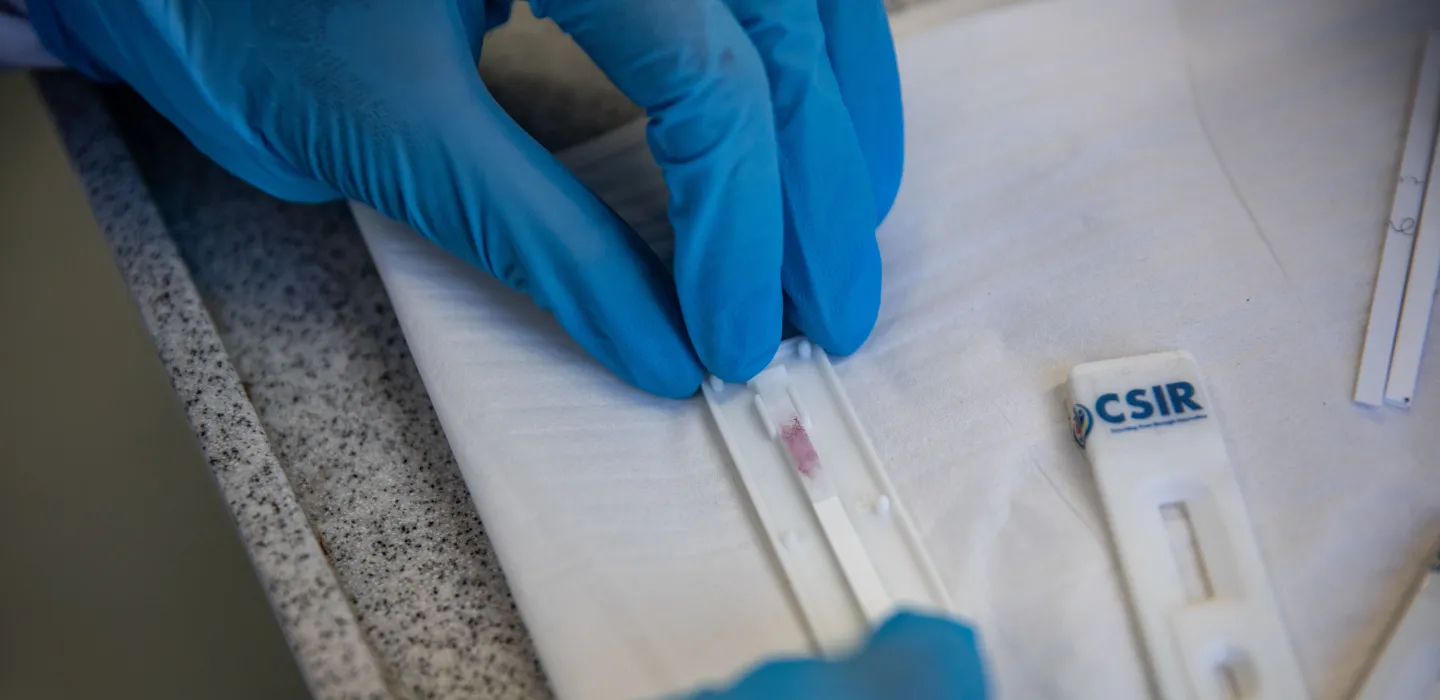
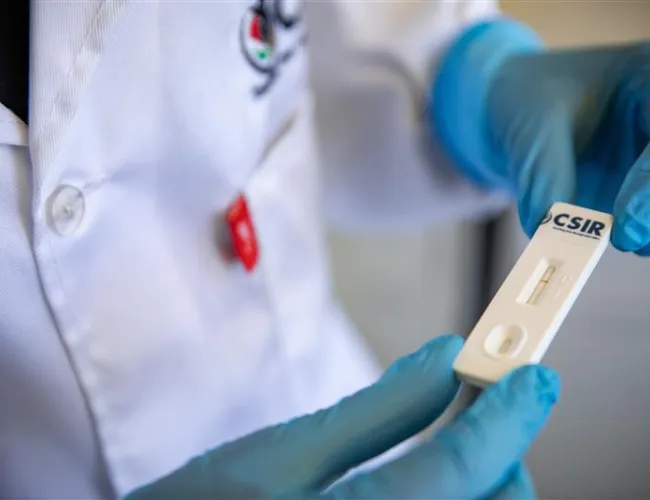
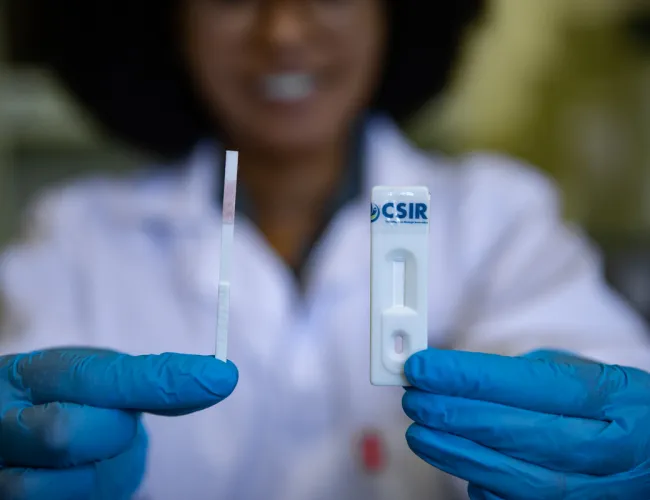
Rapid tests to screen food crops for destructive viral infections
New point-of-care virus diagnostic kits could soon help South African tomato, potato and banana farmers detect outbreaks early, preventing significant crop losses. In partnership with the Agricultural Research Council, the CSIR is developing antibody-based rapid tests for tomato spotted wilt virus, pepper ringspot virus and banana bunchy top virus. Researchers have successfully expressed the viral proteins in E. coli bacteria, with upscaling for mass spectrometry analysis underway to confirm each protein's identity. The next step will involve expressing the same viral proteins in tobacco plants. Read more.
Capabilities
We integrate multidisciplinary scientific expertise with state-of-the-art technologies to create robust, rapid and scalable diagnostic solutions tailored for Africa’s unique health challenges.
Our facilities
Our state-of-the-art laboratories support cutting-edge health and biotechnology research, diagnostics and product development. Purpose-built to accelerate innovation, they ensure the highest standards of biosafety while enabling rapid scale-up.